537-42-8
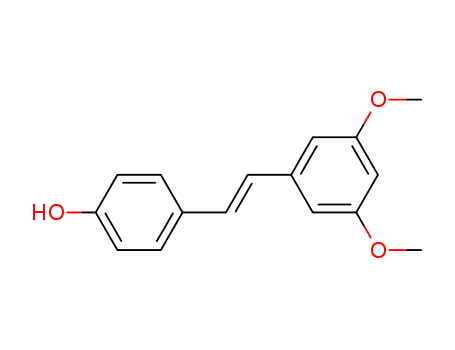
- Product Name:Pterostilbene
- Molecular Formula:C16H16O3
- Purity:99%
- Molecular Weight:256.301
Product Details
Melting Point:89-92 °C
Appearance:off-white crystalline powder
Purity:99%
Pterostilbene Good Supplier In Bulk Supply High Purity 537-42-8
- Molecular Formula:C16H16O3
- Molecular Weight:256.301
- Appearance/Colour:off-white crystalline powder
- Vapor Pressure:1.15E-07mmHg at 25°C
- Melting Point:89-92 °C
- Refractive Index:1.639
- Boiling Point:420.4 °C at 760 mmHg
- PKA:9.96±0.26(Predicted)
- Flash Point:208.1 °C
- PSA:38.69000
- Density:1.169 g/cm3
- LogP:3.57980
Pterostilbene(Cas 537-42-8) Usage
|
Preparation |
Pterostilbene was synthesized from 3,5-dimethoxybenzyl bromide and p-nitrobenzaldehyde by Witting-Hornor reaction, reduction, diazotization and hydrolysis, with a total yield of 53.9%. |
|
benefits |
Pterostilbene is a naturally-derived stilbenoid structurally related to resveratrol, with potential antioxidant, anti-inflammatory, pro-apoptotic, antineoplastic and cytoprotective activities. Pterostilbene is known to have many pharmacological benefits for the prevention and treatment of a wide variety of diseases, including ( cancer (McCormack and McFadden 2012), dyslipidaemia (Rimando et al. 2005), diabetes (Amarnath Satheesh and Pari 2006), cardiovascular degeneration (Amarnath Satheesh and Pari 2008) and pain (Hougee et al. 2005). |
|
Biological Activity |
A cell-permeable methoxylated analog of Resveratrol that displays antioxidant, anti-proliferative, and hypoglycemic properties. Appears to be a better free radical scavenger than Trolox. Moderately inhibits COX-1 & COX-2 activities (IC50 = 19.8 μM & 83.9 μM, respectively) and induces apoptosis in HL60 cells (IC50 = 70 μM). Also prevents DMBA-induced pre-neoplastic lesions (ED50 = 4.8 μM). Reported to decrease plasma glucose levels in streptozotocin-induced diabetic rats comparable to that of Metformin. |
|
Definition |
ChEBI: Pterostilbene is a stilbenol that consists of trans-stilbene bearing a hydroxy group at position 4 as well as two methoxy substituents at positions 3' and 5'. It has a role as an antioxidant, an antineoplastic agent, a neurotransmitter, a plant metabolite, an apoptosis inducer, a neuroprotective agent, an anti-inflammatory agent, a radical scavenger and a hypoglycemic agent. It is a stilbenol, a member of methoxybenzenes and a diether. It derives from a hydride of a trans-stilbene. |
InChI:InChI=1/C16H16O3/c1-18-15-9-13(10-16(11-15)19-2)4-3-12-5-7-14(17)8-6-12/h3-11,17H,1-2H3/b4-3+
537-42-8 Relevant articles
Synthesis and evaluation of resveratrol derivatives as fetal hemoglobin inducers
Andersen, Olaf Sparre,Barbieri, Karina Pereira,Bosquesi, Priscila Longhin,Carlos, Iracilda Zepone,Chelucci, Rafael Consolin,Costa, Fernando Ferreira,Dos Santos, Jean Leandro,Fernandes, Guilherme Felipe dos Santos,Lanaro, Carolina,Melchior, Aylime Castanho Bolognesi,Pavan, Aline Renata,Rusinova, Radda,de Souza, Cristiane Maria
, (2020/05/25)
Resveratrol (RVT) derivatives (10a-i) we...
Preparation method of resveratrol compound
-
Paragraph 0058; 0059; 0076; 0077; 0079; 0080, (2019/07/16)
The invention provides a preparation met...
Protective effect of piceatannol and bioactive stilbene derivatives against hypoxia-induced toxicity in H9c2 cardiomyocytes and structural elucidation as 5-LOX inhibitors
Boccellino, Mariarosaria,Donniacuo, Maria,Bruno, Ferdinando,Rinaldi, Barbara,Quagliuolo, Lucio,Ambruosi, Marika,Pace, Simona,De Rosa, Mario,Olga?, Abdurrahman,Banoglu, Erden,Alessio, Nicola,Massa, Antonio,Kahn, Haroon,Werz, Oliver,Fiorentino, Antonio,Filosa, Rosanna
, p. 637 - 647 (2019/07/31)
Stilbenes with well-known antioxidant an...
Iron-Catalyzed Nitrene Transfer Reaction of 4-Hydroxystilbenes with Aryl Azides: Synthesis of Imines via C=C Bond Cleavage
Peng, Yi,Fan, Yan-Hui,Li, Si-Yuan,Li, Bin,Xue, Jing,Deng, Qing-Hai
supporting information, p. 8389 - 8394 (2019/10/16)
C=C bond breaking to access the C=N bond...
537-42-8 Process route
-
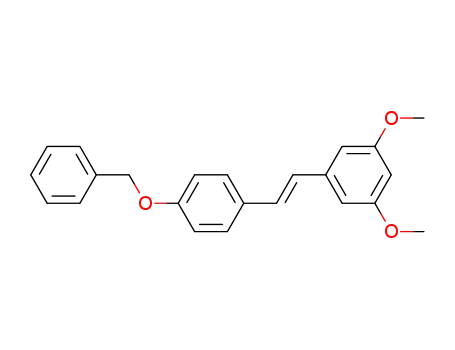
-
(E)-4'-benzyloxy-3,5-dimethoxystilbene

-
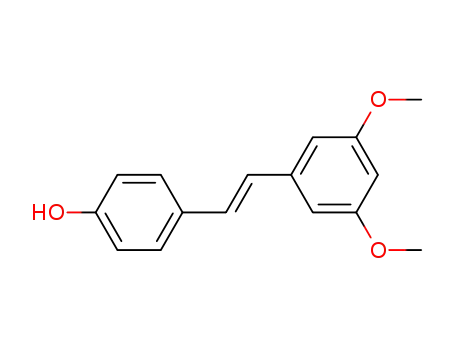
-
537-42-8,18259-15-9
3,5-dimethoxy-4'-hydroxy-trans-stilbene
| Conditions | Yield |
|---|---|
|
With
hydrogen;
In
dichloromethane;
at 20 ℃;
for 5h;
under 3750.38 Torr;
|
87% |
|
With
boron tribromide; isoascorbic acid;
In
dichloromethane;
at -78 - 20 ℃;
Inert atmosphere;
|
45% |
-
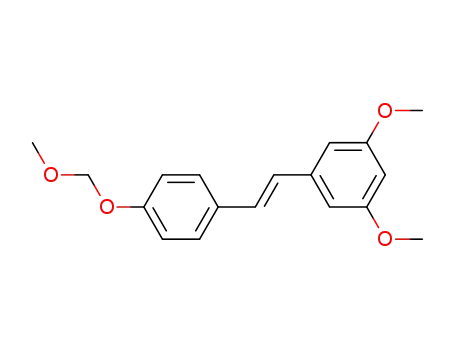
-
848487-76-3
(E)-1-(4'-(methoxymethoxy)styryl)-3,5-dimethoxybenzene

-

-
537-42-8,18259-15-9
3,5-dimethoxy-4'-hydroxy-trans-stilbene
| Conditions | Yield |
|---|---|
|
With
pyridinium p-toluenesulfonate;
In
methanol;
Heating;
|
93% |
|
With
hydrogenchloride;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 1h;
|
86% |
|
With
hydrogenchloride;
In
tetrahydrofuran; water;
at 20 ℃;
for 1h;
|
86% |
537-42-8 Upstream products
-
441351-31-1
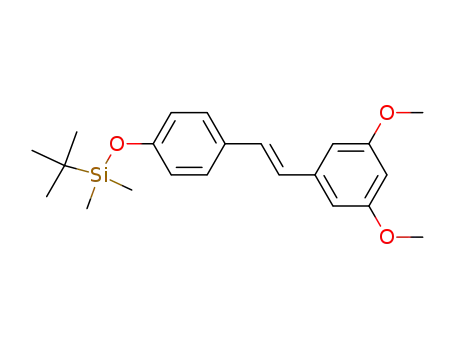
trans-3,5-dimethoxy-4'-tert-butyldimethylsilyloxystilbene
-
501-36-0
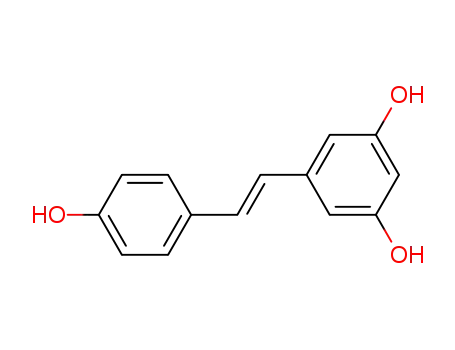
(E)-5-[2-4-(hydroxyphenyl)ethenyl]-1,3-benzenediol
-
77-78-1
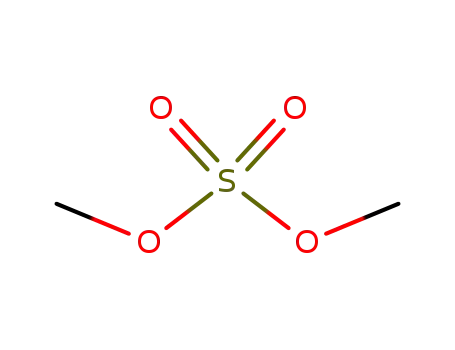
dimethyl sulfate
-
848487-76-3

(E)-1-(4'-(methoxymethoxy)styryl)-3,5-dimethoxybenzene
537-42-8 Downstream products
-
22255-22-7
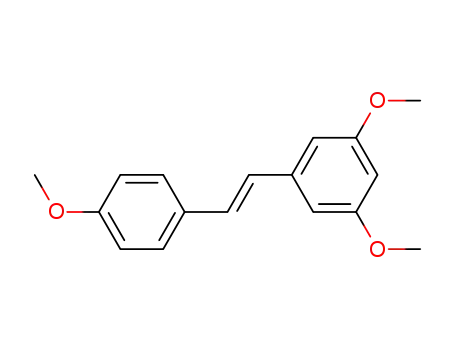
3,5,4'-trimethoxy-trans-stilbene
-
1132-21-4
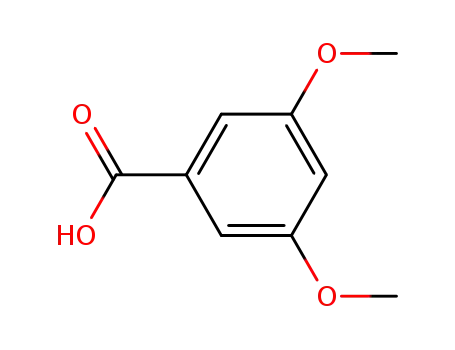
3,5-dimethoxybenzoic acid
-
1028098-06-7
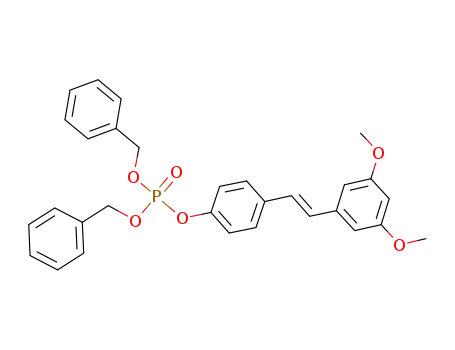
(E)-dibenzyl 4-(3,5-dimethoxystyryl)phenyl phosphate
-
116518-94-6
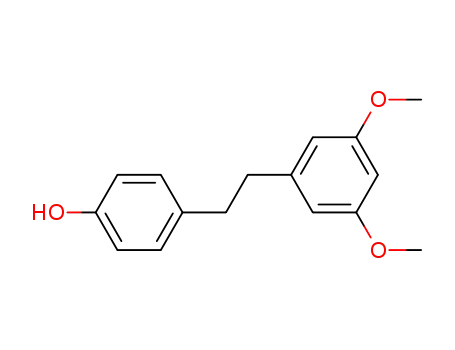
3,5-di-O-methyldihydroresveratrol
Relevant Products
-
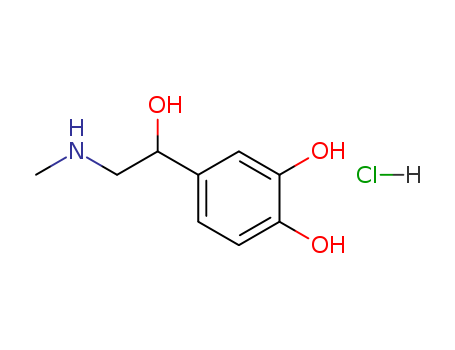
Epinephrine Hydrochloride
CAS:329-63-5
-
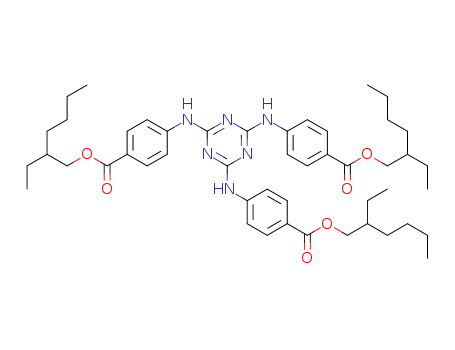
Ethylhexyl Triazone
CAS:88122-99-0
-
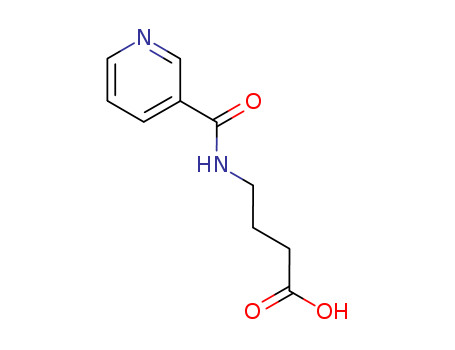
Pikamilone
CAS:34562-97-5