674-26-0
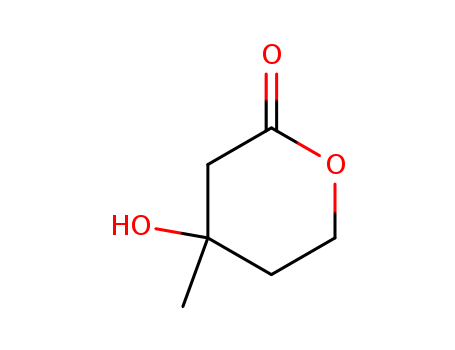
- Product Name:DL-Mevalonolactone
- Molecular Formula:C6H10 O3
- Purity:99%
- Molecular Weight:130.144
Product Details
Melting Point:28 °C(lit.)
Appearance:Moist pale yellow to off white solid
Purity:99%
Factory Export Top Purity DL-Mevalonolactone 674-26-0 In Stock
- Molecular Formula:C6H10 O3
- Molecular Weight:130.144
- Appearance/Colour:Moist pale yellow to off white solid
- Vapor Pressure:0.000662mmHg at 25°C
- Melting Point:28 °C(lit.)
- Refractive Index:n20/D 1.473(lit.)
- Boiling Point:145-150 °C5 mm Hg(lit.)
- PKA:13.57±0.20(Predicted)
- Flash Point:>230 °F
- PSA:46.53000
- Density:1.189 g/cm3
- LogP:0.07440
DL-Mevalonolactone(Cas 674-26-0) Usage
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 104, p. 5486, 1982 DOI: 10.1021/ja00384a040Synthesis, p. 719, 1974 DOI: 10.1055/s-1974-23416 |
|
Purification Methods |
Purify the lactone via the dibenzyl-ethylenediammonium salt (m 124-125o) [Hofmann et al. J Am Chem Soc 79 2316 1957], or by chromatography on paper or on a Dowex-1 (formate) column. [Bloch et al. J Biol Chem 234 2595 1959.] Store it as the N,N'-dibenzylethylenediamine (DBED) salt, or as the lactone in a sealed container at 0o. [Beilstein 18/1 V 19.] |
|
General Description |
Alkaline hydrolysis of mevalonolactone gives mevalonate. Mevalonate is a precursor of farnesyl and geranylgeranyl pyrophosphates. These pyrophosphates are required for protein prenylation. |
InChI:InChI=1/C6H10O3/c1-6(8)2-3-9-5(7)4-6/h8H,2-4H2,1H3
674-26-0 Relevant articles
MANUFACTURING METHOD OF β-HYDROXYLACTONE (METH)ACRYLIC ACID ESTER
-
Paragraph 0045; 0049-0051, (2020/01/23)
PROBLEM TO BE SOLVED: To provide a metho...
Method for preparing mevalonolactone from biosynthesized mevalonic aicd using phosphoric acid
-
Paragraph 0038; 0040; 0046, (2018/08/02)
The present invention refers to a method...
METHODS OF FORMING DIOL COMPOUNDS
-
Paragraph 0094, (2017/11/06)
Methods of forming a C4 to C7 diol compo...
PROCESSES FOR CONVERSION OF BIOLOGICALLY DERIVED MEVALONIC ACID
-
Paragraph 0094, (2016/06/13)
The invention relates to a process compr...
674-26-0 Process route
-
-
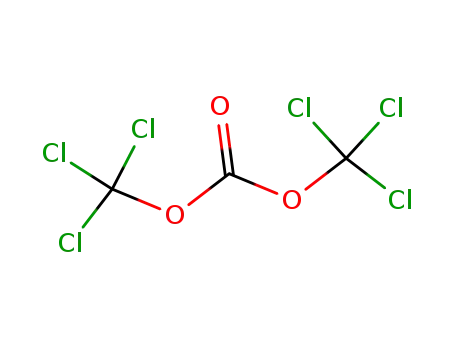
32315-10-9
bis(trichloromethyl) carbonate

-
-
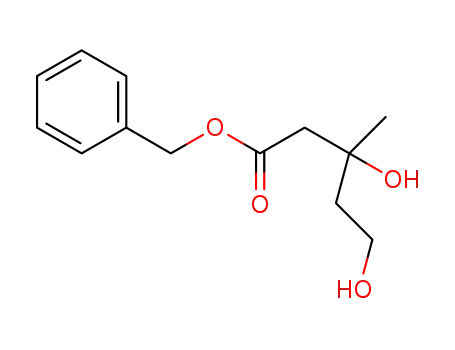
1452161-92-0
benzyl 3,5-dihydroxy-3-methylpentanoate

-
-
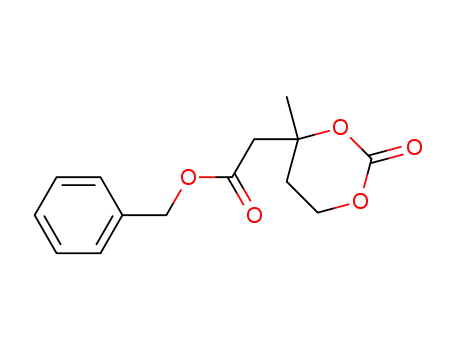
1452161-93-1
benzyl 2-(4-methyl-2-oxo-1,3-dioxan-4-yl)acetate

-
-
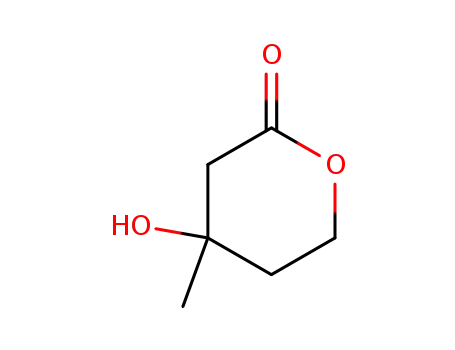
674-26-0,503-48-0
mevalonolactone
| Conditions | Yield |
|---|---|
|
With
pyridine;
In
dichloromethane;
at 0 ℃;
for 0.5h;
Inert atmosphere;
|
64% 12% |
-
-
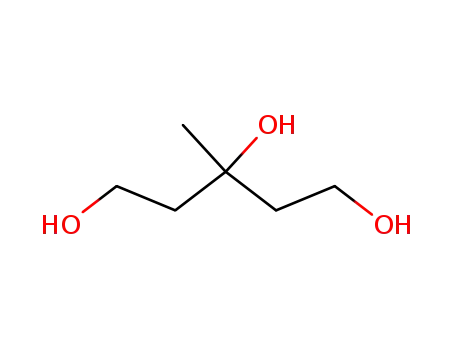
7564-64-9
3-methyl-1,3,5-pentanetriol

-

-
674-26-0,503-48-0
mevalonolactone
| Conditions | Yield |
|---|---|
|
With
pyridinium chlorochromate;
In
dichloromethane;
for 4h;
Ambient temperature;
|
92% |
|
With
aluminum oxide; sodium bromite;
In
dichloromethane;
for 1.5h;
Ambient temperature;
|
85% |
|
With
peracetic acid; sodium bromide;
In
ethyl acetate;
at 39.9 ℃;
for 2h;
|
83% |
|
With
1-methyl-1H-imidazole; [2,2]bipyridinyl; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; tetrakis(acetonitrile)copper(I) trifluoromethanesulfonate;
In
acetonitrile;
at 22 ℃;
for 6h;
|
83% |
674-26-0 Upstream products
-
463-51-4
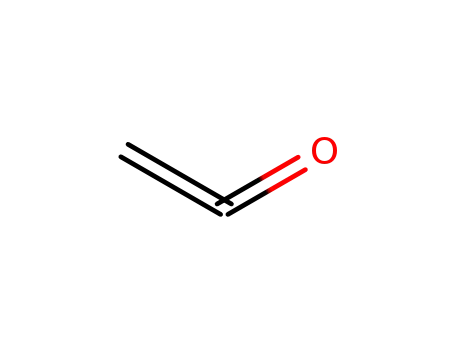
Ketene
-
10150-87-5
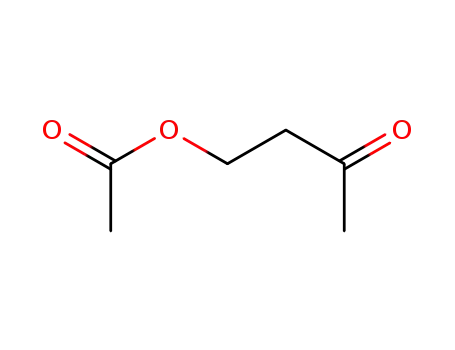
1-acetoxybutan-3-one
-
25201-40-5
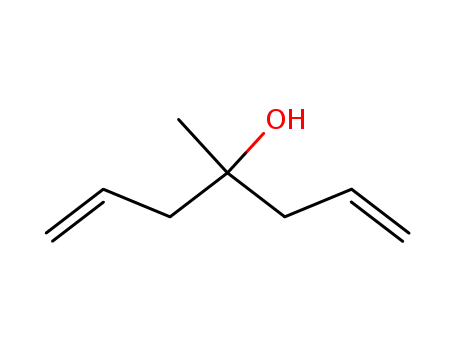
1,1-diallylethanol
-
64-19-7
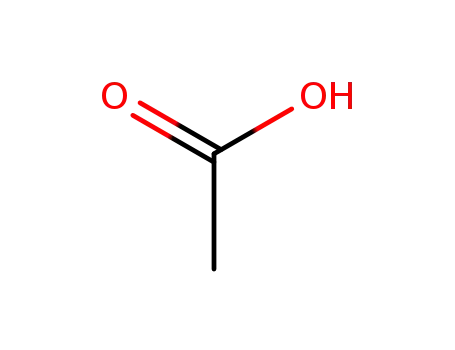
acetic acid
674-26-0 Downstream products
-
105338-90-7
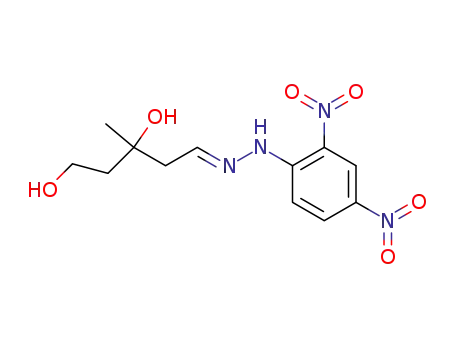
3,5-dihydroxy-3-methyl-valeraldehyde-(2,4-dinitro-phenylhydrazone)
-
5973-99-9
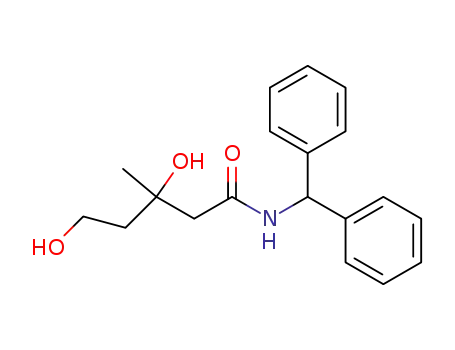
(+/-)-3,5-dihydroxy-3-methyl-valeric acid benzhydrylamide
-
7564-64-9

3-methyl-1,3,5-pentanetriol
-
72165-50-5
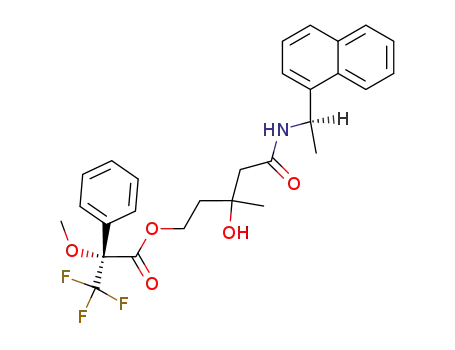
(R)-3,3,3-Trifluoro-2-methoxy-2-phenyl-propionic acid 3-hydroxy-3-methyl-4-((R)-1-naphthalen-1-yl-ethylcarbamoyl)-butyl ester
Relevant Products
-
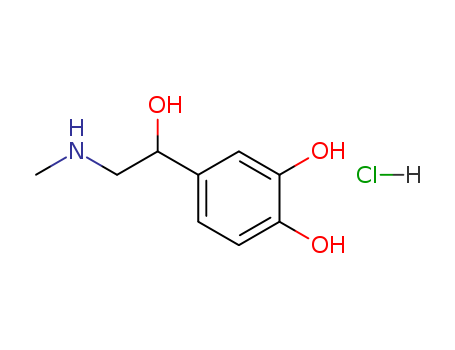
Epinephrine Hydrochloride
CAS:329-63-5
-
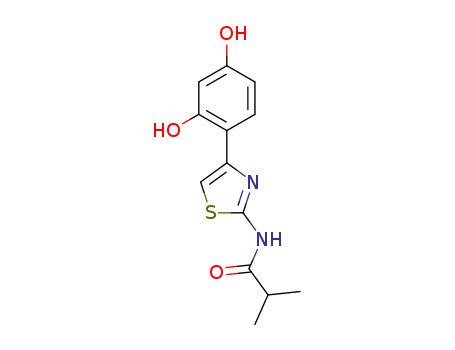
N-(4-(2,4-dihydroxyphenyl)thiazol-2-yl)isobutyramide
CAS:1428450-95-6
-
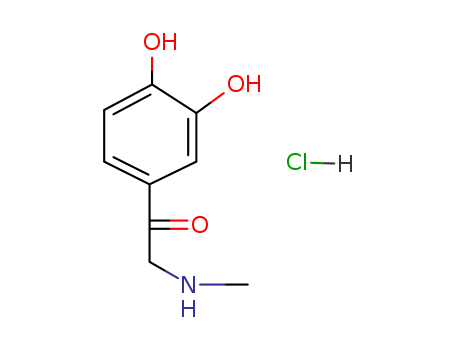
Adrenalone hydrochloride
CAS:62-13-5