489-84-9
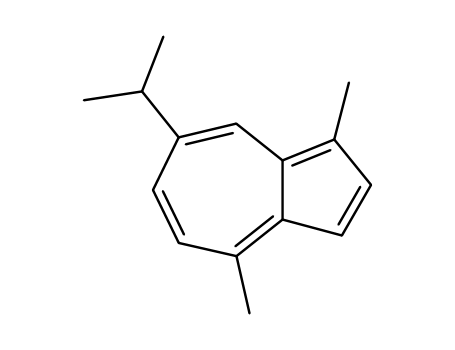
- Product Name:Guaiazulene
- Molecular Formula:C15H18
- Purity:99%
- Molecular Weight:198.308
Product Details
Melting Point:28 - 30 C
Appearance:dark blue semi-solid
Purity:99%
Reliable Quality Guaiazulene 489-84-9 Hot Sale with Chinese Manufacturer
- Molecular Formula:C15H18
- Molecular Weight:198.308
- Appearance/Colour:dark blue semi-solid
- Vapor Pressure:0.00148mmHg at 25°C
- Melting Point:28 - 30 C
- Refractive Index:1.572
- Boiling Point:153 °C7 mm Hg(lit.)
- Flash Point:113 ºC
- PSA:0.00000
- Density:0.976
- LogP:4.53160
Guaiazulene(Cas 489-84-9) Usage
| Source | Guaiazulene is a sesquiterpene. It derives from a hydride of a guaiane. Guaiazulene is a natural product found in Artemisia thuscula, Magnolia officinalis, and other organisms with data available. |
|
Therapeutic Function |
Antiinflammatory |
|
General Description |
Guaiazulene (GA) is a naturally occurring, dark blue, crystalline hydrocarbon that is derived from plants and essential oils. It's also known as azulon or 1,4-dimethyl-7-isopropylazulene. |
|
Uses |
Numerous studies have found that Guaiazulene (GA) can help to manage various conditions, including bacterial infections, tumors, immunomodulation, expectorants, diuretics, diaphoresis, ulcers, dermatitis, proliferation, and gastritis. |
InChI:InChI=1/C15H18/c1-10(2)13-7-5-11(3)14-8-6-12(4)15(14)9-13/h5-10H,1-4H3
489-84-9 Relevant articles
ALISMOL AND ALISMOXIDE, SESQUITERPENOIDS OF ALISMA RHIZOMES
Oshima, Yoshiteru,Iwakawa, Tsuneo,Hikino, Hiroshi
, p. 183 - 186 (1983)
From the crude drug 'takusha', wich is t...
Guaiazulene and related compounds: A review of current perspective on biomedical applications
Wasim Akram a 1 , Priti Tagde b c , Sakeel Ahmed d 1 , Swamita Arora b , Talha Bin Emran e f , Ahmad O. Babalghith g , Sherouk Hussein Sweilam h i , Jesus Simal-Gandara j
, Life Sciences Volume 316 , 1 March 2023, 121389
GA and its related compounds exhibit therapeutic potential in several diseases. Still, it is necessary to investigate their potential in animal models for various other ailments and establish their safety profile. They might be a good candidate to advance to clinical trials.
Reaction of azulene with 1,2-bis[4-(dimethylamino)phenyl]-1,2-ethanediol in a mixed solvent of methanol and acetonitrile in the presence of hydrochloric acid: A facile one-pot synthesis and properties of new triarylethylenes possessing an azulen-1-yl group
Takekuma, Shin-Ichi,Kaibara, Masamichi,Minematsu, Toshie,Takekuma, Hideko
experimental part, p. 4780 - 4792 (2011/08/03)
Reaction of azulene (1) with 1,2-bis[4-(...
Chemistry of (+)-aromadendrene. Part 6: Rearrangement reactions of ledene, isoledene and their epoxides
Moreno-Dorado, F. Javier,Lamers, Yvonne M. A. W.,Mironov, Grigore,Wijnberg, Joannes B. P. A.,De Groot, Aede
, p. 7743 - 7750 (2007/10/03)
The chemistry of (+)-ledene and (-)-isol...
489-84-9 Upstream products
-
110-86-1
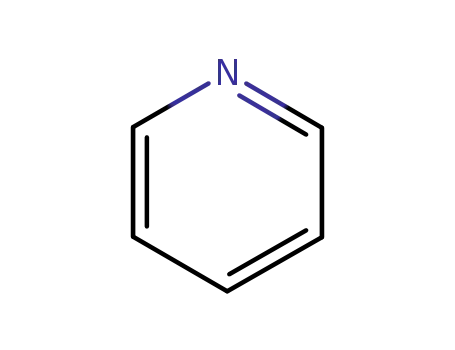
pyridine
-
98-59-9
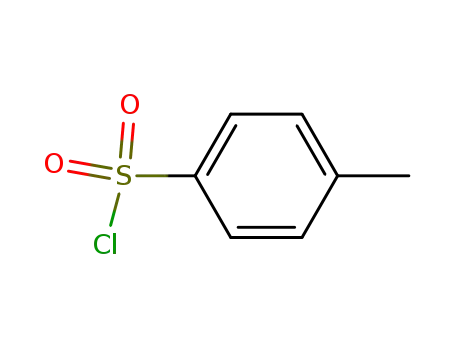
p-toluenesulfonyl chloride
-
888-21-1
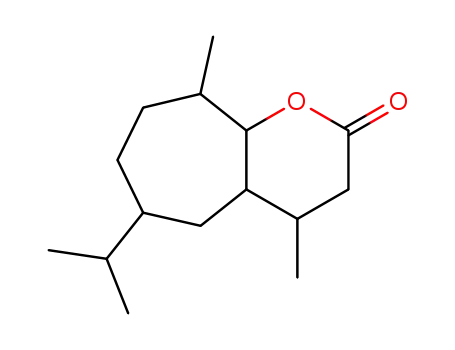
6-isopropyl-4,9-dimethyl-octahydro-cyclohepta[b]pyran-2-one
-
6894-57-1
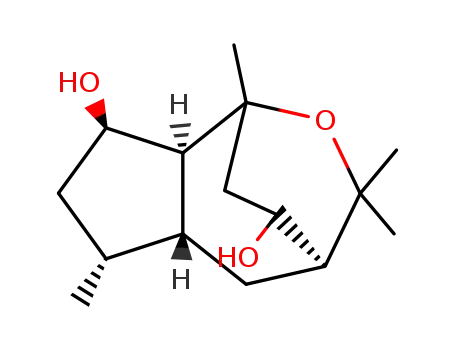
kessyl glycol
489-84-9 Downstream products
-
144-62-7
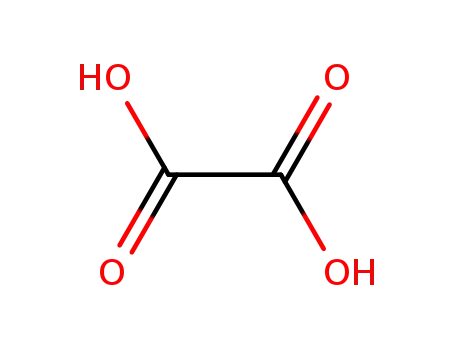
oxalic acid
-
50-00-0
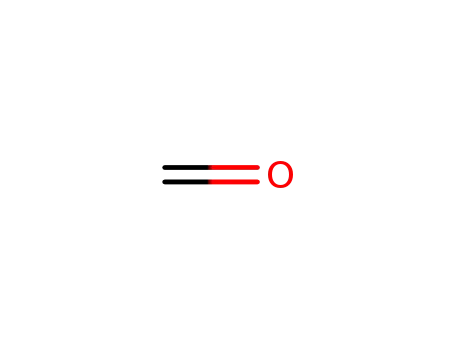
formaldehyd
-
64-18-6
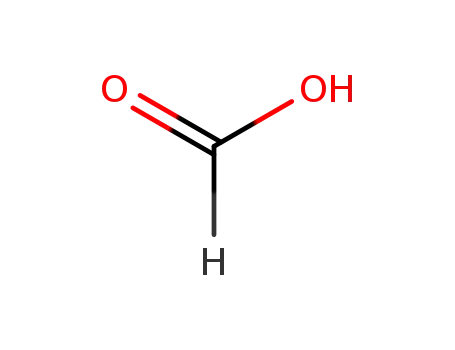
formic acid
-
64-19-7
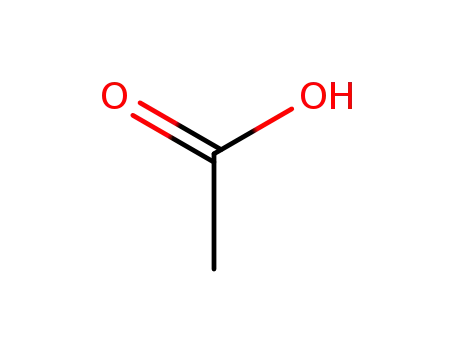
acetic acid
Relevant Products
-
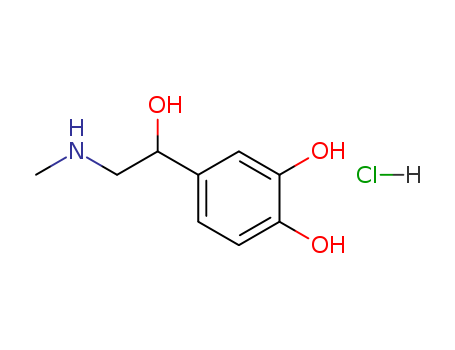
Epinephrine Hydrochloride
CAS:329-63-5
-
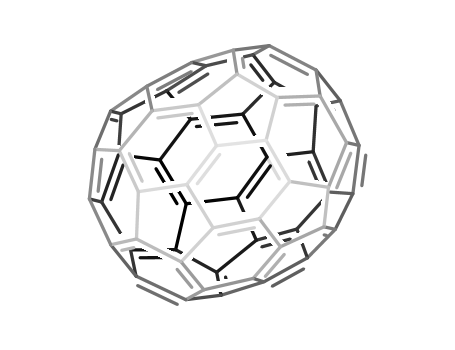
Fullerene-C60
CAS:99685-96-8
-
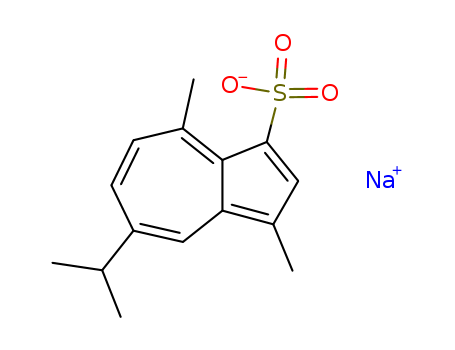
Sodium Gualenate
CAS:6223-35-4