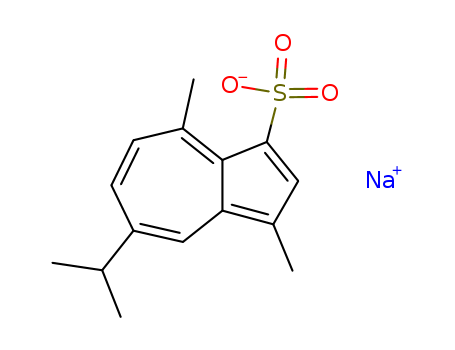
6223-35-4
- Product Name:Sodium Gualenate
- Molecular Formula:C15H17NaO3S
- Purity:99%
- Molecular Weight:300.354
Product Details
Melting Point:98°C(lit.)
Purity:99%
Factory Sells Best Quality Sodium Gualenate 6223-35-4 with USP
- Molecular Formula:C15H17NaO3S
- Molecular Weight:300.354
- Melting Point:98°C(lit.)
- PSA:65.58000
- LogP:4.51650
Sodium gualenate(Cas 6223-35-4) Usage
|
Mechanism of action |
Sodium gualenate inhibits the release of histamine from inflammatory cells through local direct action; increases the synthesis of prostaglandin E2 in the mucosa, promotes granulation formation and epithelial cell regeneration; and can reduce the activity of pepsin. |
InChI:InChI=1/C15H18O3S.Na/c1-9(2)12-6-5-10(3)15-13(8-12)11(4)7-14(15)19(16,17)18;/h5-9H,1-4H3,(H,16,17,18);/q;+1/p-1
6223-35-4 Relevant articles
Synthesis and Biological Evaluation of 3, 8-dimethyl-5-isopropylazulene Derivatives as Anti-gastric Ulcer Agent
Cao, Tingting,Li, Yong,Yang, Ziyao,Yuan, Mingxing,Li, Ying,Yang, Hongjun,Feng, Yuchuan,Yin, Shufan
, p. 264 - 271 (2016)
Recent studies showed that Guaiazulene (...
Antiretroviral (HIV-1) activity of azulene derivatives
Peet, Julia,Selyutina, Anastasia,Bredihhin, Aleksei
, p. 1653 - 1657 (2016/04/05)
The antiretroviral activity of azulene d...
N-SUBSTITUTED ISOPROPYLDIMETHYL AZULENE SULFONAMIDE DERIVATIVES, AND PREPARATION METHOD AND USE THEREOF
-
Paragraph 0108-0110, (2014/08/06)
The present invention provides an N-subs...
Synthesis and antigastric ulcer activity of novel 5-isoproyl-3,8- dimethylazulene derivatives
Zhang, Lu-Yun,Yang, Fang,Shi, Wan-Qi,Zhang, Ping,Li, Ying,Yin, Shu-Fan
, p. 5722 - 5725 (2011/10/18)
5-Isoproyl-3,8-dimethylazulene derivativ...
6223-35-4 Process route
-
- 489-84-9
7-isopropyl-1,4-dimethyl-azulene

-
- 6223-35-4
Azulon
| Conditions | Yield |
|---|---|
|
With sulfuric acid; acetic anhydride; at 20 ℃; for 2h; Cooling with ice;
|
87.5% |
|
7-isopropyl-1,4-dimethyl-azulene; With sulfuric acid; acetic anhydride; at 20 ℃; for 4h;
With sodium hydroxide; In water;
|
57% |
|
Multi-step reaction with 2 steps
1: sulfuric acid; acetic anhydride / 3 h / 20 °C
2: sodium hydroxide
With sulfuric acid; acetic anhydride; sodium hydroxide;
|
|
|
7-isopropyl-1,4-dimethyl-azulene; With sulfuric acid; acetic anhydride; at 20 ℃; for 3h;
With sodium hydroxide;
|
-
- 16915-32-5
5-isopropyl-3,8-dimethyl-azulene-1-sulfonic acid

-
- 6223-35-4
Azulon
| Conditions | Yield |
|---|---|
|
With sodium hydroxide;
|
6223-35-4 Upstream products
-
489-84-9
7-isopropyl-1,4-dimethyl-azulene
-
16915-32-5
5-isopropyl-3,8-dimethyl-azulene-1-sulfonic acid
6223-35-4 Downstream products
-
1338350-97-2
N-cyclohexyl-3,8-dimethyl-5-isopropyl-1-azulene sulfonamide
-
1338350-98-3
N-benzyl-3,8-dimethyl-5-isopropyl-1-azulene sulfonamide
-
1338350-99-4
N-(4-chlorophenyl)-3,8-dimethyl-5-isopropyl-1-azulene sulfonamide
-
1338351-00-0
N-(4-aminophenyl)-3,8-dimethyl-5-isopropyl-1-azulene sulfonamide
Relevant Products
-
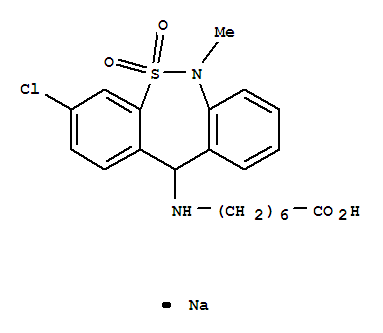
Tianeptine sodium salt
CAS:30123-17-2
-
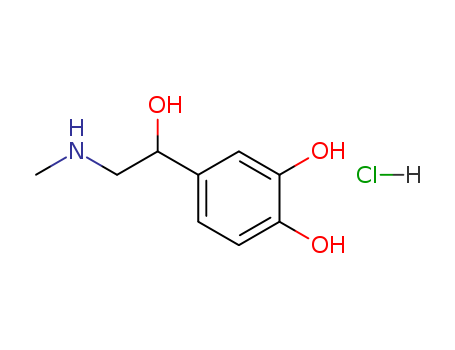
Epinephrine Hydrochloride
CAS:329-63-5
-
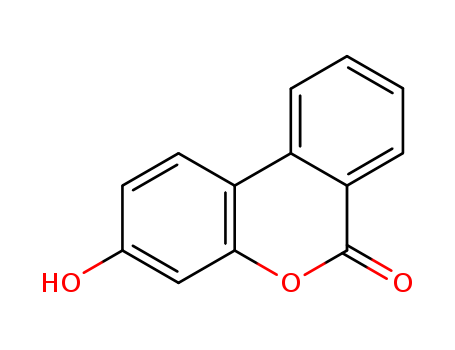
Urolithin B
CAS:1139-83-9