38398-32-2
- Product Name:GANAXOLONE
- Molecular Formula:C22H36 O2
- Purity:99%
- Molecular Weight:332.52
Product Details
Melting Point:190-192°
Purity:99%
Quality Manufacturer Supply High Purity 99% GANAXOLONE 38398-32-2 with Reasonable Price
- Molecular Formula:C22H36 O2
- Molecular Weight:332.52
- Vapor Pressure:2.19E-09mmHg at 25°C
- Melting Point:190-192°
- Boiling Point:434.8°Cat760mmHg
- PKA:15.18±0.70(Predicted)
- Flash Point:185.4°C
- PSA:37.30000
- Density:1.036g/cm3
- LogP:4.98530
GANAXOLONE(Cas 38398-32-2) Usage
|
Biological Activity |
Potent positive allosteric modulator of GABA A receptors. Enhances GABA-evoked chloride currents in Xenopus oocytes expressing GABA A receptors (EC 50 values are 94, 122 and 213 nM for α 2 β 1 γ 2 L , α 3 β 1 γ 2 L and α 1 β 1 γ 2 L receptors respectively). Exerts anticonvulsive effects in a broad range of animal seizure models. |
|
Uses |
Ganaxolone is used to treat seizures associated with cyclin-dependent kinase-like 5 deficiency disorder (CDKL5; an inherited condition that begins in early childhood and causes seizures and developmental delays) in adults and children 2 years of age and older. |
InChI:InChI=1/C22H36O2/c1-14(23)17-7-8-18-16-6-5-15-13-20(2,24)11-12-21(15,3)19(16)9-10-22(17,18)4/h15-19,24H,5-13H2,1-4H3/t15-,16-,17+,18-,19-,20+,21-,22+/m0/s1
38398-32-2 Relevant articles
NEUROSTEROID COMPOUNDS AND METHODS FOR THEIR PREPARATION AND USE IN TREATING CENTRAL NERVOUS SYSTEM DISORDERS
-
Paragraph 0103; 0105; 0109, (2019/11/12)
Described herein is the chemical structu...
Safety and efficacy of ganaxolone in patients with CDKL5 deficiency disorder: results from the double-blind phase of a randomised, placebo-controlled, phase 3 trial
Elia M Pestana Knight, MD Sam Amin, MD Prof Nadia Bahi-Buisson, MD Prof Tim A Benke, MD Prof J Helen Cross, MD Scott T Demarest, MD
Volume 21, ISSUE 5, P417-427, May 2022
Ganaxolone significantly reduced the frequency of CDD-associated seizures compared with placebo and was generally well tolerated. Results from what is, to our knowledge, the first controlled trial in CDD suggest a potential treatment benefit for ganaxolone. Long-term treatment is being assessed in the ongoing open-label extension phase of this trial.
38398-32-2 Process route
-
- 566-65-4
dihydroprogesterone

-
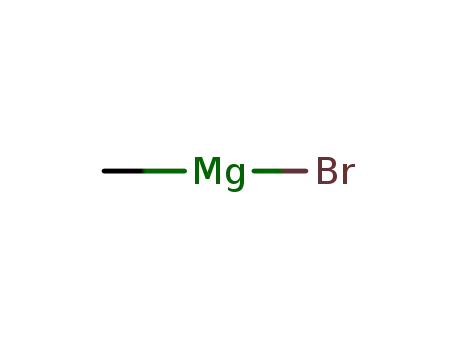
- 75-16-1
methylmagnesium bromide

-
- 38398-32-2
ganaxolone
| Conditions | Yield |
|---|---|
|
methylmagnesium bromide; With iron(III) chloride; lithium chloride; In tetrahydrofuran; diethyl ether; at -35 - -30 ℃; for 0.5h;
dihydroprogesterone; In tetrahydrofuran; diethyl ether; at -20 - -15 ℃; for 2h;
|
81% |
|
In toluene; at -70 ℃; for 2h;
|
0.3 g |
-
![1-((2’R,8R,10S,13S,14S,17S)-10,13-dimethylhexadecahydrospiro[cyclopenta[a]phenanthrene-3,2’-oxiran]-17-yl)ethanone](/upload/2024/8/3ceb179c-30c4-43d1-8feb-7a3a0ee286ae.png)
- 148256-45-5
1-((2’R,8R,10S,13S,14S,17S)-10,13-dimethylhexadecahydrospiro[cyclopenta[a]phenanthrene-3,2’-oxiran]-17-yl)ethanone

-

- 38398-32-2
ganaxolone
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: 67 percent / NaI, glacial AcOH / tetrahydrofuran; methanol / a) reflux, 6 h, b) RT, overnight
2: 51 percent / H2, NaOAc / 5percent Pd/C / methanol; tetrahydrofuran / 18 h
With hydrogen; sodium acetate; acetic acid; sodium iodide; palladium on activated charcoal; In tetrahydrofuran; methanol;
|
|
|
1-((2’R,8R,10S,13S,14S,17S)-10,13-dimethylhexadecahydrospiro[cyclopenta[a]phenanthrene-3,2’-oxiran]-17-yl)ethanone; With acetic acid; sodium iodide; In tetrahydrofuran; methanol; at 65 ℃; for 2h;
With palladium 10% on activated carbon; hydrogen; sodium acetate; In methanol; under 2585.81 Torr;
|
0.522 g |
38398-32-2 Upstream products
-
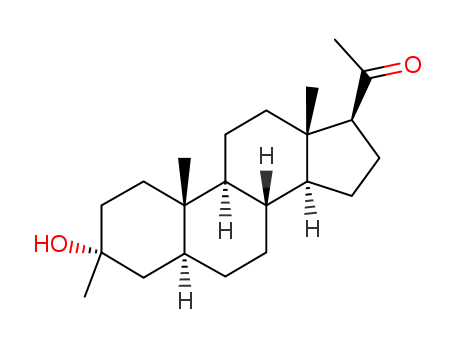
162882-87-3
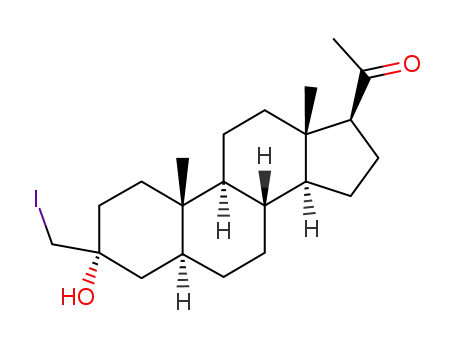
1-((3R,5S,8R,9S,10S,13S,14S,17S)-3-Hydroxy-3-iodomethyl-10,13-dimethyl-hexadecahydro-cyclopenta[a]phenanthren-17-yl)-ethanone
-
55571-86-3
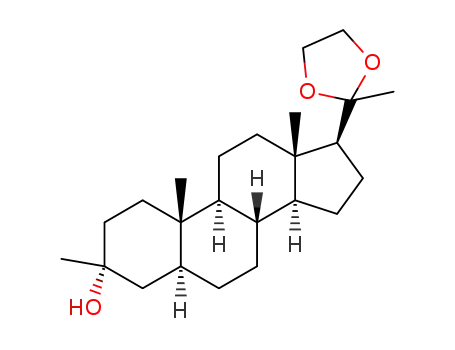
20,20-ethylenedioxy-3β-methyl-5α-pregnan-3α-ol
-
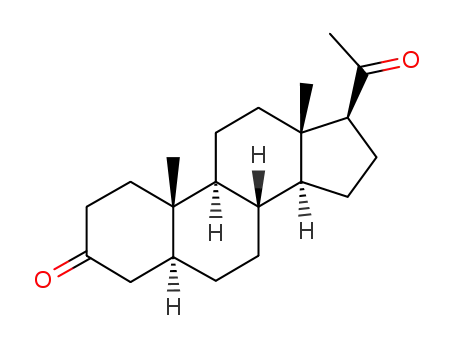
566-65-4

dihydroprogesterone
-
148256-45-5
1-((2’R,8R,10S,13S,14S,17S)-10,13-dimethylhexadecahydrospiro[cyclopenta[a]phenanthrene-3,2’-oxiran]-17-yl)ethanone
Relevant Products
-
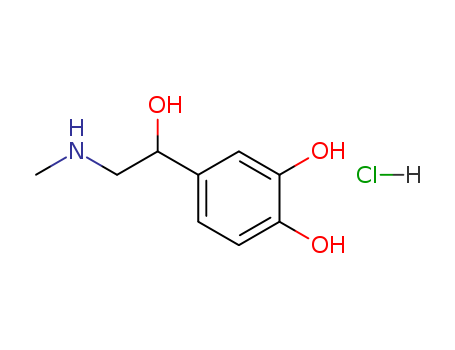
Epinephrine Hydrochloride
CAS:329-63-5
-
Vitamin K2(Menaquinone-4)
CAS:11032-49-8
-
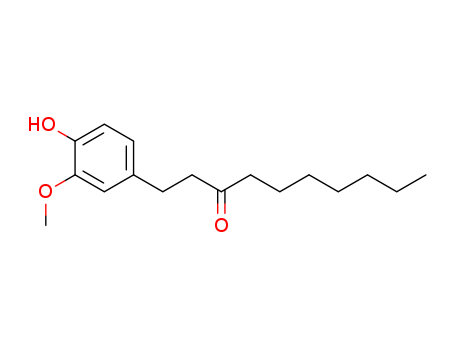
6-Paradol
CAS:27113-22-0