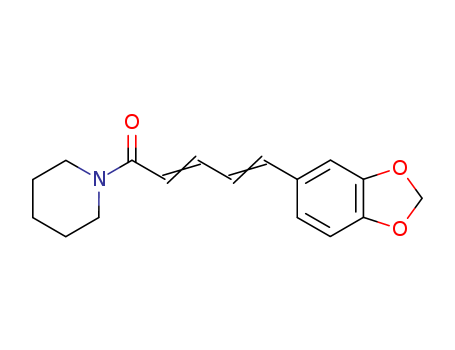
94-62-2
- Product Name:Piperine
- Molecular Formula:C17H19NO3
- Purity:99%
- Molecular Weight:285.343
Product Details
Melting Point:131-135 °C(lit.)
Appearance:slightly yellow powder
Purity:99%
Perfect Factory Offer Excellent quality Piperine 94-62-2 with Safe Shipping
- Molecular Formula:C17H19NO3
- Molecular Weight:285.343
- Appearance/Colour:slightly yellow powder
- Vapor Pressure:0mmHg at 25°C
- Melting Point:131-135 °C(lit.)
- Refractive Index:1.615
- Boiling Point:498.5 °C at 760 mmHg
- PKA:12.22(at 18℃)
- Flash Point:255.3 °C
- PSA:38.77000
- Density:1.211 g/cm3
- LogP:2.93510
Piperine(Cas 94-62-2) Usage
|
History |
Hans Christian Oersted (1777 1851) isolated piperine from black pepper in 1819 and published his findings in 1820. Oersted extracted a resin from pepper plants with alcohol, formed a soluble saltby adding hydrochloric acid with alcohol, and then separated piperine from solution by precipitation and distillation. In 1882, Leopold Rügheimer (1850 1917) synthesized piperine from piperinic acid chloride and piperidine. The complete synthesis of piperine was reported in 1894 by Albert Ladenburg (1842 1911) and M. Scholtz. Ladenburg and Scholtz used piperonal (C8H6O3) and acetaldehyde (CH3CHO) to produce piperinic acid (C12H10O4), which was then reacted with thionyl chloride (COCl2) and piperidine (C5H11N) to produce piperine. |
|
Preparation |
From piperoyl chloride and piperidine. |
|
Safety Profile |
Poison by ingestion and intraperitoneal routes. An experimental teratogen. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of NOx. |
|
Purification Methods |
Piperine crystallises as light yellow crystals from EtOH or EtOAc (m 132o), aqueous EtOH (m 128-129o), Et2O (m129o), or*benzene/ligroin. [Beilstein 20 H 79, 20 I 23, 20 II 53, 20 III/IV 1341, 20/3 V 469.] |
InChI:InChI=1/C17H19NO3/c19-17(18-10-4-1-5-11-18)7-3-2-6-14-8-9-15-16(12-14)21-13-20-15/h2-3,6-9,12H,1,4-5,10-11,13H2/b6-2-,7-3+
94-62-2 Relevant articles
The development of novel cytochrome P450 2J2 (CYP2J2) inhibitor and the underlying interaction between inhibitor and CYP2J2
Tian, Xiangge,Zhou, Meirong,Ning, Jing,Deng, Xiaopeng,Feng, Lei,Huang, Huilian,Yao, Dahong,Ma, Xiaochi
, p. 737 - 748 (2021/03/16)
Human Cytochrome P450 2J2 (CYP2J2) as an...
Identification and optimization of piperine analogues as neuroprotective agents for the treatment of Parkinson's disease via the activation of Nrf2/keap1 pathway
Cai, Xiaoying,Chen, Lijuan,Hong, Feng,Kuang, Shuang,Li, Yan,Ma, Xu,Qi, Wenyan,Shi, Mingsong,Wang, Lun,Xu, Ruiling,Xue, Linlin,Ye, Haoyu,Zhang, Ruijia
, (2020/05/11)
Parkinson's disease (PD) is a slowly pro...
Piperine derivative as well as preparation method and application thereof
-
Paragraph 0142; 0178-0184, (2020/05/08)
The invention provides a piperine deriva...
Vicinal difluorination as a C=C surrogate: An analog of piperine with enhanced solubility, photostability, and acetylcholinesterase inhibitory activity
Lizarme-Salas, Yuvixza,Ariawan, Alexandra Daryl,Ratnayake, Ranjala,Luesch, Hendrik,Finch, Angela,Hunter, Luke
, p. 2663 - 2670 (2020/12/31)
Piperine, a natural product derived from...
94-62-2 Process route
-
-
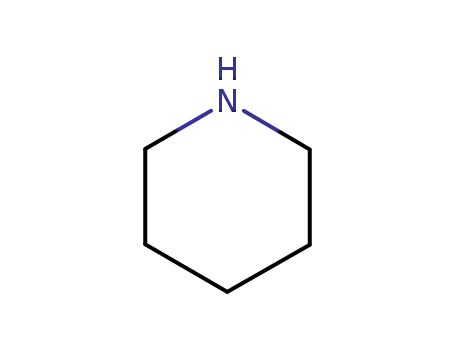
110-89-4
piperidine

-
-
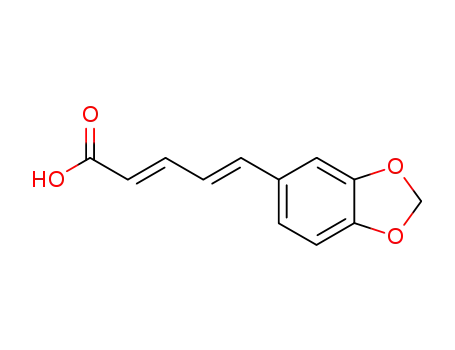
136-72-1
piperic acid

-
-
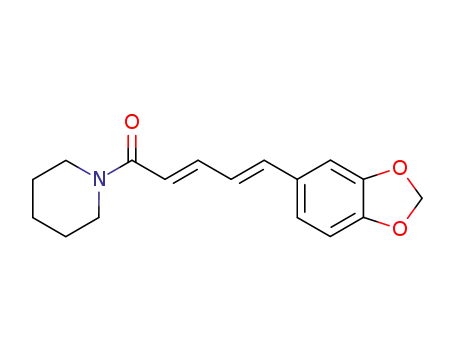
94-62-2,7780-20-3
Piperine
| Conditions | Yield |
|---|---|
|
piperic acid;
With
thionyl chloride;
In
dichloromethane;
at 20 ℃;
Schlenk technique;
piperidine;
In
dichloromethane;
at 20 ℃;
for 1h;
stereoselective reaction;
Schlenk technique;
Inert atmosphere;
|
95% |
|
With
O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 8h;
|
93% |
|
With
N,N-bis[2-oxo-3-oxazolidinyl]phosphorodiamidic chloride; triethylamine;
In
dichloromethane;
at 20 - 25 ℃;
for 1h;
|
90% |
|
With
dmap; dicyclohexyl-carbodiimide;
|
72% |
|
With
dmap; dicyclohexyl-carbodiimide;
In
dichloromethane;
for 18h;
Inert atmosphere;
|
71% |
-
-
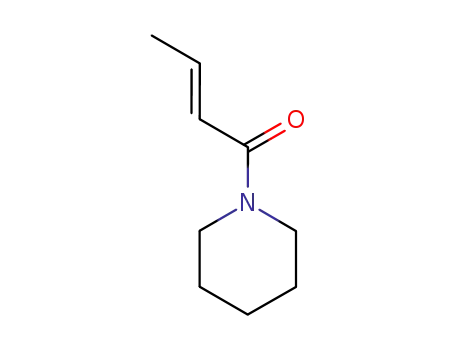
50838-22-7
1-crotonoylpiperidine

-
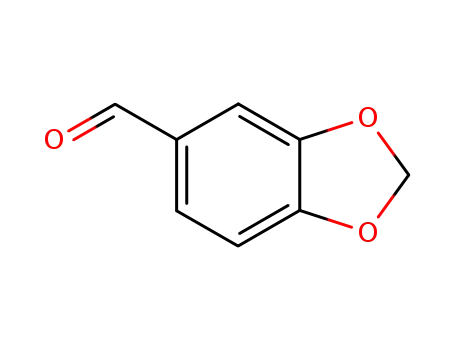
-
120-57-0,30024-74-9
piperonal

-

-
94-62-2,7780-20-3
Piperine
| Conditions | Yield |
|---|---|
|
With
Aliquat 336; potassium carbonate;
In
toluene;
at 90 ℃;
for 10h;
|
95% |
|
With
potassium hydroxide; N-benzyl-N,N,N-triethylammonium chloride;
In
dimethyl sulfoxide;
1) 15 min, 25 deg C 2) 2 h, 60-65 deg C;
|
88% |
|
piperonal;
With
sodium hydroxide;
In
water; dimethyl sulfoxide;
for 0.5h;
1-crotonoylpiperidine;
In
water; dimethyl sulfoxide;
at 20 ℃;
|
75.3% |
|
With
potassium hydroxide; N-benzyl-N,N,N-triethylammonium chloride;
In
dimethyl sulfoxide;
at 60 - 65 ℃;
for 2h;
Product distribution;
various solvents;
|
|
|
With
N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide;
In
water; dimethyl sulfoxide;
at 25 - 30 ℃;
|
89 g |
94-62-2 Upstream products
-
104683-09-2
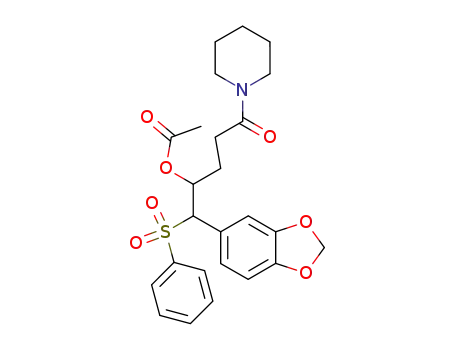
Acetic acid 1-(benzenesulfonyl-benzo[1,3]dioxol-5-yl-methyl)-4-oxo-4-piperidin-1-yl-butyl ester
-
110-89-4

piperidine
-
20850-43-5
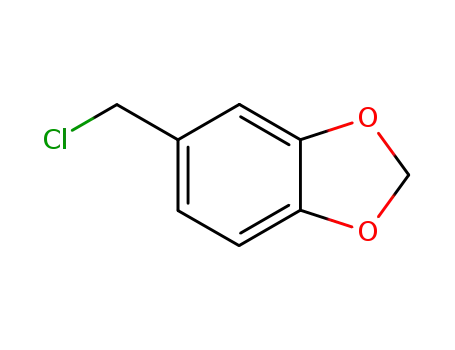
5-(chloromethyl)-1,3-benzodioxole
-
495-76-1
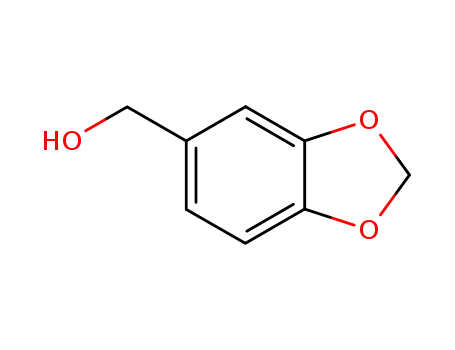
piperonol
94-62-2 Downstream products
-
91869-08-8
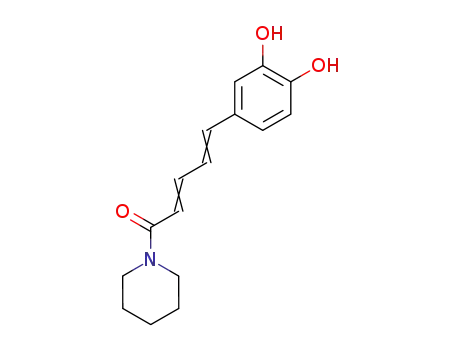
5-(3,4-Dihydroxyphenyl)-2,4-pentadiensaeure-piperidid
-
5285-18-7
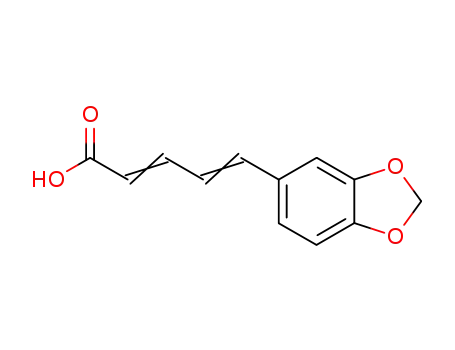
5-(1,3-benzodioxol-5-yl)-2,4-pentadienoic acid
-
91869-12-4
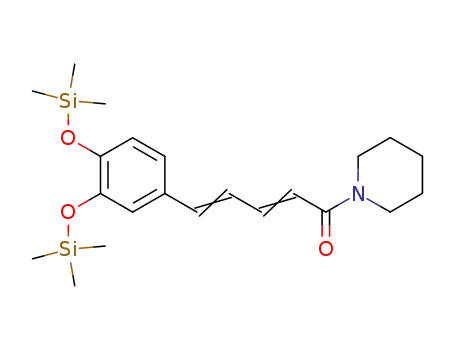
1-<5-<3,4-Bis(trimethylsilyloxy)phenyl>-1-oxo-2,4-pentadienyl>piperidin
-
23434-88-0
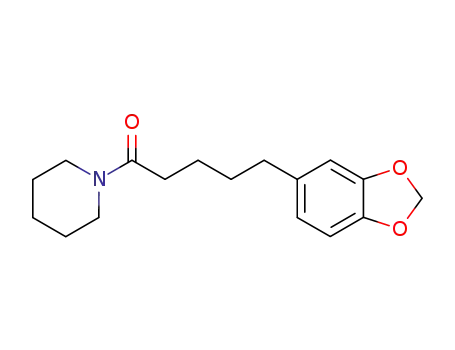
tetrahydropiperine
Relevant Products
-
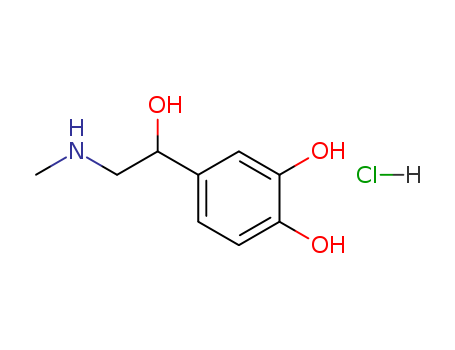
Epinephrine Hydrochloride
CAS:329-63-5
-
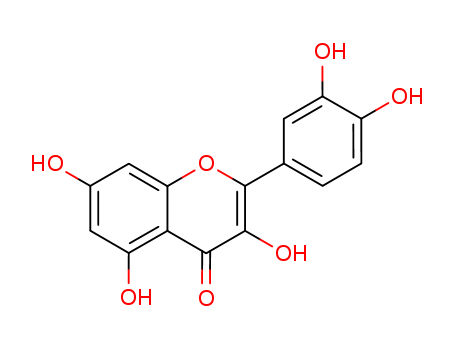
Quercetin
CAS:117-39-5
-
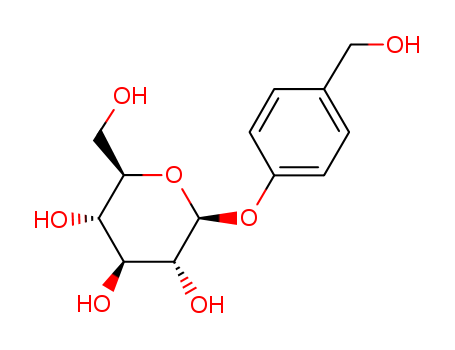
Gastrodin
CAS:62499-27-8