Is NMNH replacing NMN? Or is it a forced creation?
10/23/2025 11:51:28
Nicotinamide mononucleotide (NMN) is a derivative of vitamin B3 (niacin) and one of several precursors to nicotinamide adenine dinucleotide (NAD+), a compound essential for cellular processes in the body. As people age, NAD+ levels tend to drop significantly, which scientists believe may contribute to age-related health conditions.
Research suggests that NMN supplements can increase NAD+ levels, help reduce age-related inflammation, support cardiovascular health, and enhance brain function. Despite its potential anti-aging effects, the U.S. Food and Drug Administration (FDA) recently banned the sale of NMN as a supplement. The ban came after the agency had already allowed companies to sell it, sparking outrage among natural health advocates and the dietary supplement industry. Did you know that the FDA has banned NMN as a dietary supplement: reasons and how it happened?
However, another compound — dihydronicotinamide mononucleotide (NMNH) — is being sold on Amazon and other retail sites as an alternative to NMN, a move by supplement companies that could raise the same legal issues as NMN. Although more research is needed to determine its safety, experts say NMNH may work better than NMN as an NAD+ boosting compound. What is NMNH, and is it better than NMN? NMNH is a non-natural molecule that can be converted to NMNH by some artificial means. Its safety and stability in the human body have not been studied and confirmed.
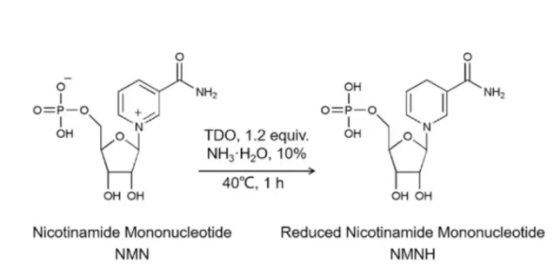
According to Dr. Robert Verkerk, founder and executive scientific director of the Natural Health Alliance, NMNH is the reduced form of NMN. It works similarly to NMN in that they are both NAD+ precursors, but there appear to be some differences in the enzyme pathways involved.“Some recent laboratory studies suggest that NMNH may be more effective than NMN in increasing NAD+ levels in the body,” Dr. Verkerk said.
Dr. Andrew Shao, senior vice president of global regulatory and scientific affairs at ChromaDex, told CNN that NMNH may be 5-10 times more potent than NMN in animals. While research is limited, a 2021 study found that NMNH was a better NAD+ enhancer than NMN in both laboratory studies and studies of living organisms. Results of a study published in 2021 also showed that NMNH increased NAD+ levels faster and more than NMN or nicotinamide riboside (NR), another NAD+ precursor. The scientists found that mice given the compound had a rapid and sustained surge in NAD+ detected in their blood. Is NMNH safe?
Dr. Shao said the safety of oral NMNH is unclear because there are no studies on its safety in animals or humans. Studies examining its NAD-boosting effects have been conducted by injecting NMNH. Therefore, its safety, effectiveness, and stability in oral form are not clear. "It's still early days for this molecule," said Dr. Verkerk. “Although there have been no safety signals, it is reasonable to assume that the safety profile of NMNH will be similar to that of NMN due to the molecular similarities.” Legal Issues with NMNH When a company wants to market a new ingredient as a supplement, it must voluntarily submit a New Dietary Ingredient (NDI) application to the FDA. Although the FDA initially accepted NMN as a dietary supplement, it later reversed its decision and essentially deemed that NMN was not legal to market. The FDA stated that because NMN has been authorized for investigation as a new drug, it cannot be marketed in the U.S.
NMN and other technical issues may eventually affect NMNH, which is currently being sold online and in stores.“Although the FDA’s decision only applies to NMN, NMNH is chemically different from NMN, which makes it a new dietary ingredient that requires notification to the FDA,” Shao explained. “As of today, no NDI notifications have been submitted to the FDA for NMNH. This means that NMNH on the market is technically not legal.” Dr. Verkerk agreed that the FDA may consider NMNH a new dietary ingredient. Companies that want to market NMNH will have to comply with the requirements of the NDI application in order to be considered legal. “Supplement companies also use the Generally Recognized As Safe (GRAS) process to bring new products to market.”Will NMNH be banned like NMN? Dr. Verkerk says what happened to NMN could also happen to NMNH if a pharmaceutical company filed an Investigational New Drug Application (IND) before NMNH was marketed as a supplement. “The frustrating thing about this legal loophole is that INDs are confidential, so pharmaceutical companies can hold onto their INDs for years while the supplement industry paves the way for an ingredient, and then pharmaceutical companies come in with their INDs and drive out supplement competition, effectively creating a monopoly,” Dr. Verkerk says.Shao thinks it’s unlikely that the FDA will ban NMNH at this stage. “However, given that there are no published studies examining the safety or efficacy of oral NMNH and no company has submitted an Investigational New Drug Application (NDI) to the FDA, NMNH is still in the research phase and has a long way to go,” Shao says. Bottom LineThe FDA does not “approve” dietary supplements before a product is marketed—including supplements like NMNH. Instead, it monitors supplements by inspecting manufacturing facilities and following reports of adverse events after the products are sold to consumers. So until scientists learn more about the safety and effectiveness of NMNH, consumers should consider exercising caution when buying supplements online or in retail stores.




