Fisetin, a natural product in fruits and vegetables, delays aging
10/22/2025 14:37:35
Fisetin is a flavonoid found in fruits and vegetables, including strawberries, apples and cucumbers. Recent studies have shown that the antioxidant has powerful anti-aging activity and can prevent the replication of damaged DNA.
In October 2018, researchers from the Center for Molecular Medicine and Aging at the Scripps Research Institute and the Mayo Clinic found that fisetin can maintain health and extend the lifespan of mice. The relevant results were published in the journal EBioMedicine under the title "Fisetin is a senotherapeutic that extends health and lifespan".

Of the 10 flavonoids tested, fisetin was the most effective anti-aging agent. Acute or intermittent treatment of premature and elderly mice with fisetin reduced aging markers in multiple tissues. Fisetin reduced senescence of a subset of cells in mouse and human adipose tissue, demonstrating cell type specificity. Administration of fisetin to wild-type mice in late life restored tissue homeostasis, reduced age-related pathology, and extended median and maximum lifespan. This study identified the flavonoid polyphenol fisetin as a more potent therapeutic activity than quercetin in cultured cells.
Furthermore, fisetin had potent therapeutic activity in vivo. Acute or intermittent treatment of progeroid and aged wild-type mice with fisetin reduced markers of senescence in multiple tissues and in some cell types in adipose tissue. Importantly, chronic administration of fisetin to wild-type mice late in life improved tissue homeostasis, suppressed age-related pathologies, and extended median and maximum lifespan. This result is similar to a recent report on the D ± Q combination, documenting for the first time that senolytics can extend healthspan and lifespan with few side effects, even when initiated late in life.
 1. Identification of fisetin as a putative anti-senogenic agent.
1. Identification of fisetin as a putative anti-senogenic agent.
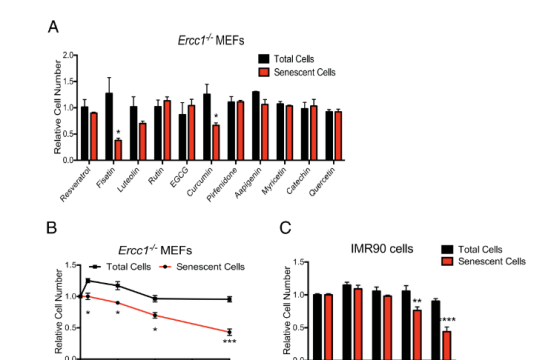
A senescent cell model was established using primary MEF cells from Ercc1−/− mice, established under 3% O2, and then transferred to 20% O2 for three passages to induce senescence. SA-ß-gal activity was measured using the fluorescent substrate C12FDG. Fisetin was the most effective of the screened panel of flavonoids for reducing the proportion of SA-ß-gal-positive MEFs (Figure 1A). Fisetin reduced senescence in MEFs and IMR90 cells in a dose-dependent manner.
2. Intermittent treatment of progeroid mice with fisetin reduces the burden of senescent cells To test the therapeutic activity of fisetin in vivo, progeroid Ercc1−/Δ mice carrying a p16ink4a-luciferase reporter transgene were used. Ercc1−/Δ; p16ink4a-luciferase mice were fed a standard Teklad 2020 mouse diet supplemented with or without 500 ppm (500 mg/kg) of fisetin ad libitum (approximately 60 mg/kg per day). Mice were intermittently fed the fisetin diet between 6 and 8 weeks and 12-14 weeks. Whole body luciferase activity was measured before starting the fisetin diet and then weekly thereafter. Animals in both treatment groups had equivalent luciferase signals prior to administration of the experimental diet. Dietary fisetin significantly suppressed the p16ink4a-luciferase luciferase signal in Ercc1−/Δ mice (Fig. 2A, B). After the initiation of the fisetin diet, the luciferase signal was lower at every time point (Fig. 2B-C). Notably, during the 4-week period without exposure to fisetin, the p16Ink4a expression level in the fisetin-treated mice remained significantly lower.
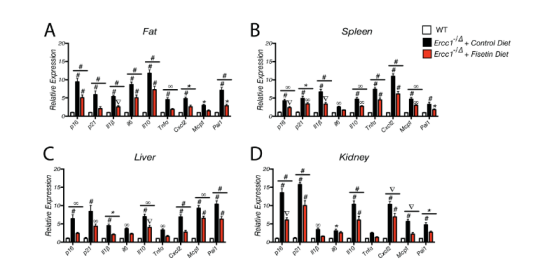
3. Long-term fisetin treatment reduces senescence in progeroid mice To validate the imaging data, Ercc1-/Δ mice were treated with a 500 ppm fisetin diet for 10 weeks starting at 10 weeks of age, and tissues were then collected for measurement of multiple senescence markers, including p16Ink4a and p21Cip1 and SASP factors. As shown in Fig. 3A-D, p16Ink4a and p21Cip1 mRNA and SASP markers were significantly elevated in the fat, spleen, liver, and kidneys of Ercc1-/Δ mice compared with age-matched WT mice. Fisetin significantly reduced the expression of senescence and SASP markers in all tissues. Similarly, the expression of p16 Ink4a, p 21 Cip1, and SASP factors was reduced in peripheral blood CD3 + T cells (Figure 3E). In addition, it was determined that fisetin reduced oxidative stress in the liver by measuring the lipid peroxidation product 4-hydroxynonenal (HNE) adducts and the increase in the ratio of reduced glutathione to oxidized glutathione.
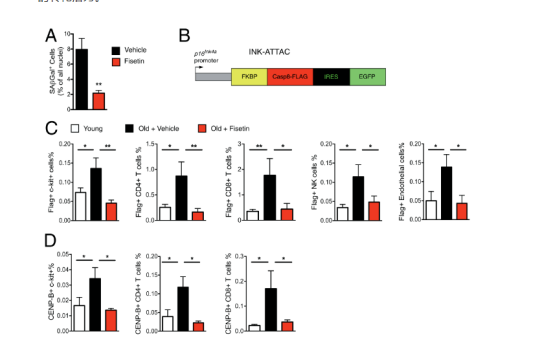
4.Acute fisetin treatment reduces the burden of senescent cells in aged wild-type mice and human explants To further confirm the data obtained in progeroid mice, naturally aged C57BL/6 mice and different methods of detecting tissue senescence were used. Mice aged 22 to 24 months were gavaged with 100 mg/kg fisetin for 5 consecutive days. Mice were sacrificed 3 days after the last dose and the number of SA-β-gal+ cells present in the inguinal fat was determined by staining tissue sections to measure SA-β-gal activity. Adipose tissue was chosen for analysis because there are clear markers of senescence in mouse models including upregulation of the SASP, and a significant increase in the proportion of senescent cells in the tissue, including senescent immune cells such as T cells and endothelial cells and macrophages. Short-term treatment with fisetin significantly reduced the proportion of senescent cells in white adipose tissue (WAT) (Figure 4A). To determine which cells are senescent in WAT and which cell types are eliminated by fisetin, CyTOF analysis was performed on subcutaneous adipose tissue from aged INK-ATTAC mice expressing Flag-tagged FKBP-Casp8 protein from the p16 Ink4a promoter (Figure 4B). The Flag tag enables the identification of senescent (p16 Ink4a expressing) cells using an anti-Flag antibody. CyTOF analysis revealed a significant increase in the proportion of senescent cells in the adipose of aged mice compared to young mice and identified these cells as mesenchymal stem/progenitor cells, T lymphocytes, natural killer cells, and endothelial cells (Figure 4C). Short-term treatment with fisetin resulted in a significant reduction in the proportion of senescent cells in these populations (Figure 4C). Fisetin reduced the proportion of p16Ink4a-expressing cells, c-Kit+ stem/progenitor cells, CD4+ and CD8+ T cells, NK-1.1+ NK cells, and CD146+CD31+ endothelial cells (Figure 4C). To confirm senescence of these cell populations, CENP-B protein was measured by CyTOF (Figure 4D). CENP-B binds centromeric satellite DNA, which is expanded in senescent cells. The proportion of CENP-B+ cells in WAT was significantly increased in aged mice compared to young mice and was suppressed by treating mice with a short course of fisetin, as was the case with p16Ink4a-expressing/FLAG+ cells. To determine whether fisetin could also reduce senescence in human adipose tissue, omental adipose explants removed during surgery were treated with fisetin ex vivo. Tissue explants were treated with 20 μM fisetin for 48 h, washed and cultured for an additional 24 h, and then SASP factors were measured by multiplex protein analysis. Fisetin treatment resulted in a significant decrease in the percentage of SA-ß-gal-positive cells (Fig. 4E) and expression of the SASP factors IL-6, IL-8, and MCP-1 in human WAT (Fig. 4F). These data support the translational potential of fisetin to reduce senescent cell burden and associated inflammation.
5. Late-life intervention with fisetin in aged wild-type mice extends healthspan and lifespan To determine whether fisetin-mediated clearance of senescent cells affects healthspan or lifespan in mice, WT f1 C57BL/6:FVB mice were fed a diet containing 500 ppm fisetin starting at 85 weeks (roughly equivalent to 75 years in humans). This resulted in an increase in median and maximum lifespan (Fig. 5A,B). Serum amylase and alanine aminotransferase (ALT) were significantly reduced in aged WT mice fed a fisetin-supplemented diet, consistent with improved pancreatic and liver homeostasis (Fig. 5C). Brain, kidney, liver, lung, and forepaw tissue sections were stained with hematoxylin and eosin and evaluated by a veterinary pathologist. Age-related pathologies were scored using the Geriatric Pathology Grading Platform, and several tissues in the fisetin diet group had reduced age-related pathology compared to the control diet (Figure 5D). Representative images of kidney sections illustrate an example of this in Figure 5E. Similar to progeroid mice, fisetin reduced the expression of senescence and SASP markers in multiple tissues in aged WT mice exposed to oral fisetin (Figure 5F-I). In addition, there was a reduction in the expression of senescence and SASP factors in peripheral CD3+ T cells (Figure 5J). Circulating MCP-1 (SASP factor) levels were also reduced (Figure 5K). Finally, fisetin reduced oxidative stress in the liver of aged WT mice (Figure 5L-M).




